News
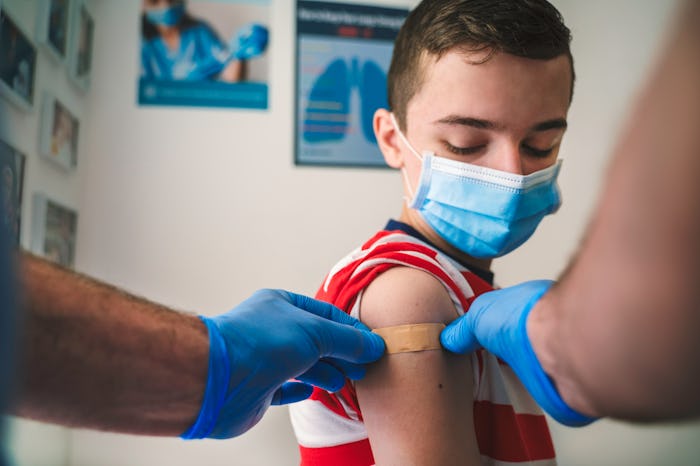
Moderna Says Its COVID-19 Vaccine Is Safe & Effective In Kids As Young As 12
No cases of COVID were observed in pediatric trial participants who received two doses of the vaccine, suggesting 100% efficiency.
Moderna’s COVID-19 vaccine has been found to be safe and effective in children ages 12 to 18, the biotechnology company announced Tuesday. According to the vaccine maker, no cases of COVID-19 were observed in participants of Moderna’s TeenCOVE study who received two doses of the vaccine, suggesting a vaccine efficacy of 100%. Moderna has said it plans to follow in Pfizer’s footsteps and ask the Food and Drug Administration (FDA) to expand the emergency authorization of its COVID-19 vaccine to include use in children as young as 12 years old.
“We are encouraged that mRNA-1273 was highly effective at preventing COVID-19 in adolescents,” Moderna CEO Stéphane Bancel said in a statement released Tuesday by the biotechnology company. “It is particularly exciting to see that the Moderna COVID-19 vaccine can prevent SARS-CoV-2 infection.
According to Moderna, 3,732 children ranging in age from 12 to 18 took part in their randomized TeenCOVE study. While no cases of COVID-19 that involved at least two symptoms were observed in fully vaccinated participants, four cases of the virus were observed in the placebo group. These results are consistent with a vaccine efficacy of 100% in adolescents beginning two weeks after the second dose was administered, Moderna said.
In an effort to adjust for children’s lower incidence rate of COVID-19, Moderna’s TeenCOVE study also evaluated the vaccine’s efficacy using a CDC definition of COVID-19 that included more mild cases such as those where only one symptom is present. Under these qualifications, a vaccine efficacy of 93% was observed in adolescent study participants following the first dose of Moderna.
Bancel said Moderna would soon submit the results of its TeenCOVE study to the FDA in hopes it will expand use of the vaccine to children 12 and up. Currently, Moderna’s COVID-19 vaccine is authorized for use only in individuals 18 and older.
“We will submit these results to the U.S. FDA and regulators globally in early June and request authorization,” Bancel said Tuesday. “We remain committed to doing our part to help end the COVID-19 pandemic.”
In a press release issued Tuesday, Moderna said its COVID-19 vaccine was “generally well tolerated” by TeenCOVE participants and had been found to have a safety profile similar to that found in its adult trials. The most common local adverse event was reported to be injection site pain while the most common systemic adverse events following the second dose were reported to be headache, fatigue, myalgia, and chills. However, the majority of these adverse events were reported to be mild or moderate, the vaccine maker noted.
In an effort to continue to accrue information about the vaccine’s safety, Moderna has said it will continue following and monitoring all adolescent participants for a full 12 months after their second injection.
Earlier this month, the FDA cleared Pfizer-BioNTech’s COVID-19 vaccine, which had already been authorized for individuals aged 16 and up, for use in 12- to 15-year-olds. Expanding Moderna’s COVID-19 vaccine for use in kids ages 12 and up, however, could significantly boost children’s access to vaccines ahead of the start of the 2021-2022 school year.
If you think you’re showing symptoms of coronavirus, which include fever, shortness of breath, and cough, call your doctor before going to get tested. If you’re anxious about the virus’s spread in your community, visit the CDC for up-to-date information and resources, or seek out mental health support. You can find all of Romper’s parents + coronavirus coverage here.